Tropospheric Chemistry
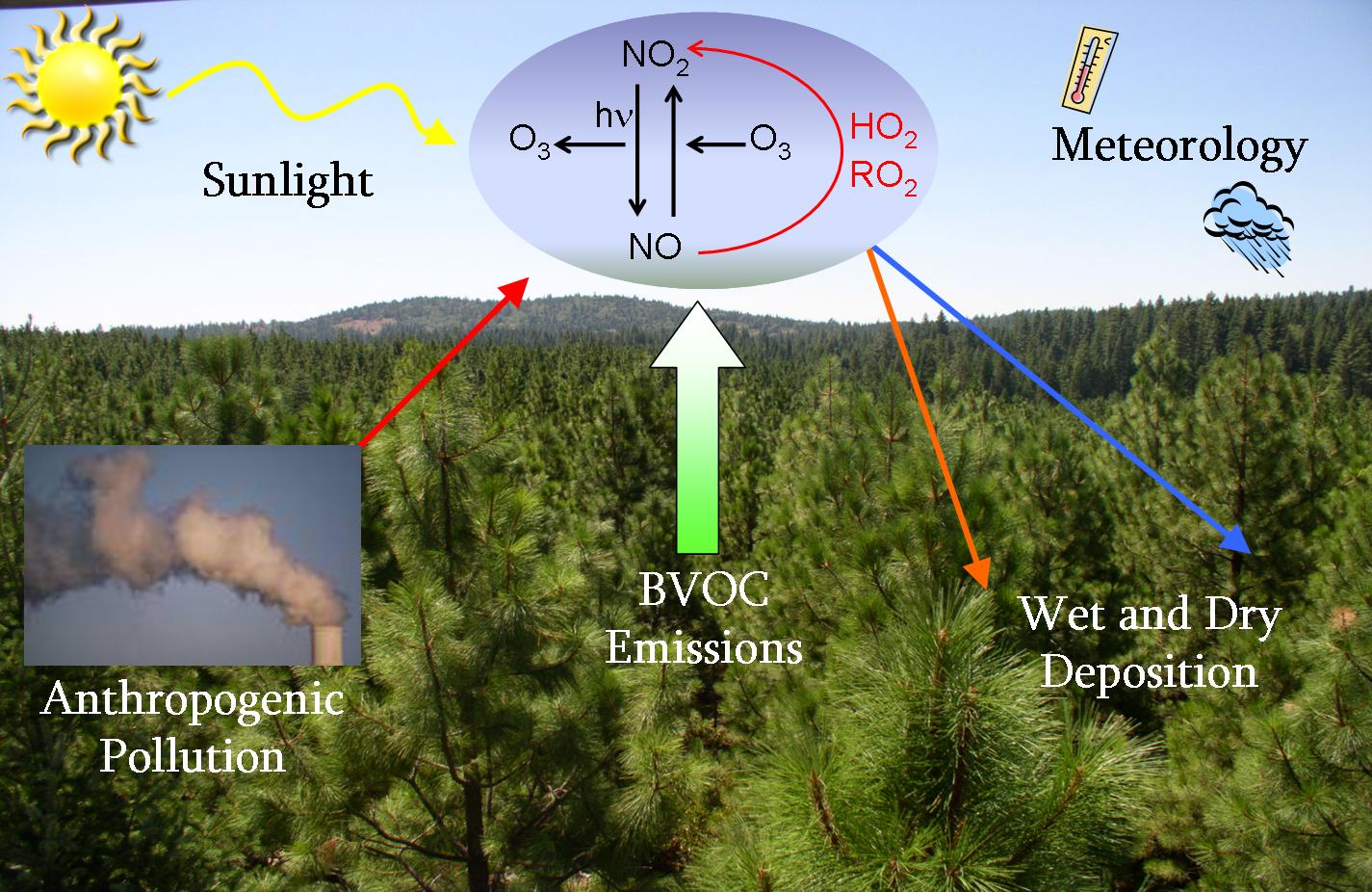
 Nitric oxide (NO) is a key ingredient of the atmosphere. It plays the central role in controlling the rate of oxidative chemistry. When present together with reactive organic compounds, reactions involving NO produce air pollution. In both cities and remote regions, NO controls the rate of formation of the premier oxidant of the atmosphere, the hydroxy radical (OH) from its photochemical cousins, the peroxy radicals (HO2 and organic analogs, RO2). Our laboratory focuses on largely unknown region of the map of oxidative chemistry where “here be dragons” – the region where NO no longer dominates the reactions with HO2 and RO2. Using new analytical methods, we have discovered and quantified new photochemical pathways that alter our understanding of how the low NO chemistry alter oxidative capacity and organic aerosol generation. These pathways, including atmospheric autoxidation, are becoming ever more prominent as NOx levels decline rapidly across North America.
Nitric oxide (NO) is a key ingredient of the atmosphere. It plays the central role in controlling the rate of oxidative chemistry. When present together with reactive organic compounds, reactions involving NO produce air pollution. In both cities and remote regions, NO controls the rate of formation of the premier oxidant of the atmosphere, the hydroxy radical (OH) from its photochemical cousins, the peroxy radicals (HO2 and organic analogs, RO2). Our laboratory focuses on largely unknown region of the map of oxidative chemistry where “here be dragons” – the region where NO no longer dominates the reactions with HO2 and RO2. Using new analytical methods, we have discovered and quantified new photochemical pathways that alter our understanding of how the low NO chemistry alter oxidative capacity and organic aerosol generation. These pathways, including atmospheric autoxidation, are becoming ever more prominent as NOx levels decline rapidly across North America.
Atmospheric Chamber
The majority of our laboratory work takes place in atmospheric chambers, which allow us to isolate and study photochemical reactions of gaseous and particulate pollutants. We use both the two large (20 cubic meter) chambers present in the Seinfeld lab, as well as our 1 cubic meter “mini chamber”. Results from these chamber studies are often used to support data collected during field studies we take part in.
Recent Chamber Study Publications:
- Stereoselectivity in Atmospheric Autoxidation. Møller et al., J. Phys. Chem. Lett., 2019
- Unimolecular Reactions of Peroxy Radicals Formed in the Oxidation of α-pinene and β-pinene by Hydroxyl Radicals. Xu et al., J. Phys Chem. A, 2019
- Intramolecular Hydrogen Shift Chemistry of Hydroperoxy-Substituted Peroxy Radicals. Praske et al., J. Phys. Chem A, 2019
- Kinetics and Product Yields of the OH Initiated Oxidation of Hydroxymethyl Hydroperoxide. Allen et al., J Phys. Chem. A, 2018
- Atmospheric autoxidation is increasingly important in urban and suburban North America. Praske et al., Proc. Natl. Acad. Sci., 2018.
- Isoprene Peroxy Radical Dynamics. Teng, et al., J. Am. Chem. Soc., 2017.
Instrumentation
GC-HRToF-CIMS
 The utilization of clustering ion chemistry (e.g., CF3O–, I–, H+), in conjunction with chemical ionization mass spectrometry (CIMS), has allowed for low fragmentation sampling of important oxygenated volatile organic compounds (OVOCs) considered too fragile to be detected otherwise. However, typical CIMS techniques cannot distinguish between isomeric products, which often causing ambiguity and uncertainty in the data sets they provide. To combat this, we have coupled low pressure gas chromatography (GC) to a high resolution time-of-flight (HRToF) CIMS. Combined, this field deployable instrument is capable of providing isomer-resolved measurements of OVOCs in the ambient air.
The utilization of clustering ion chemistry (e.g., CF3O–, I–, H+), in conjunction with chemical ionization mass spectrometry (CIMS), has allowed for low fragmentation sampling of important oxygenated volatile organic compounds (OVOCs) considered too fragile to be detected otherwise. However, typical CIMS techniques cannot distinguish between isomeric products, which often causing ambiguity and uncertainty in the data sets they provide. To combat this, we have coupled low pressure gas chromatography (GC) to a high resolution time-of-flight (HRToF) CIMS. Combined, this field deployable instrument is capable of providing isomer-resolved measurements of OVOCs in the ambient air.
Reference Paper:
- Low-pressure gas chromatography with chemical ionization mass spectrometry for quantification of multifunctional organic compounds in the atmosphere, Vasquez et al., Atmos. Meas. Tech., 2018.
CIT-CIMS
 The CIT-CIMS instrument was originally composed of a single mass analyzer CIMS (S-CIMS) and developed for use on the NASA ER-2 aircraft. Following the 2000 SOLVE campaign, it was retrofitted to fly on the NASA DC8 aircraft. The S-CIMS instrument has recently been upgraded from a quadrupole to a compact time-of-flight (cToF) CIMS, guaranteeing high temporal resolution (1 Hz or faster) and simultaneous measurement of a wide mass range. In addition to the cToF-CIMS, we have also flown a tandem CIMS (or triple quadrupole CIMS, hereafter “Triple”) instrument since INTEX-B (2006). The Triple provides parent-daughter mass analysis, enabling measurement of compounds that cannot be quantified by the cToF-CIMS due to mass interferences. Both mass spectrometers use a CF3O–, which was originally used due to its selective detection of HNO3. CF3O– has since been found to be ideal for measurements with SO2, organic acids, organic nitrates and peroxides.
The CIT-CIMS instrument was originally composed of a single mass analyzer CIMS (S-CIMS) and developed for use on the NASA ER-2 aircraft. Following the 2000 SOLVE campaign, it was retrofitted to fly on the NASA DC8 aircraft. The S-CIMS instrument has recently been upgraded from a quadrupole to a compact time-of-flight (cToF) CIMS, guaranteeing high temporal resolution (1 Hz or faster) and simultaneous measurement of a wide mass range. In addition to the cToF-CIMS, we have also flown a tandem CIMS (or triple quadrupole CIMS, hereafter “Triple”) instrument since INTEX-B (2006). The Triple provides parent-daughter mass analysis, enabling measurement of compounds that cannot be quantified by the cToF-CIMS due to mass interferences. Both mass spectrometers use a CF3O–, which was originally used due to its selective detection of HNO3. CF3O– has since been found to be ideal for measurements with SO2, organic acids, organic nitrates and peroxides.
Reference Papers:
-
- Measurement of gas-phase hydroperoxides by chemical ionization mass spectrometry. Crounse et al., Anal. Chem., 2006.
- Chemical ionization tandem mass spectrometer for the in situ measurement of methyl hydrogen peroxide St. Clair et al., Rev. Sci. Instrum., 2010.
Recent Field Campaigns
FIREX-AQ, 2019
Fire Influence on Regional to Global Environments Experiment – Air Quality Study
Instrument: CIT-CIMS
Platform: NASA DC8 Aircraft
Participants: Hannah Allen, Krystal Vasquez, Lu Xu and John Crounse
ATom, 2016-2018
Atmospheric Tomography Mission
Instrument: CIT-CIMS
Platform: NASA DC8 Aircraft
Participants: Hannah Allen, Michelle Kim and John Crounse
PROPHET-AMOS, 2016
Program for Research on Oxidants; Photochemistry, Emissions and Transport – Atmospheric Measurements of Oxidants in Summer
Instrument: GC-HRToF-CIMS
Platform: PROPHET Research Tower
Participants: Hannah Allen, Eric Praske, Krystal Vasquez and John Crounse
KORUS-AQ, 2016
Korea‐United States Air Quality Study
Instrument: CIT-CIMS
Platform: NASA DC8 Aircraft
Participants: Alex Teng, Michelle Kim, and John Crounse